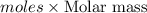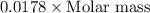Chemistry, 07.04.2020 22:58, alyssahomeworkneeds
Given the following information: 1.6 g of an unknown monoprotic acid (HA) required 50.80 mL of a 0.35 M NaOH solution to reach the equivalence point, calculate the molar mass (g/mol) of the acid. Enter the value ONLY. Do not include the units.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Do you know the correct answer?
Given the following information: 1.6 g of an unknown monoprotic acid (HA) required 50.80 mL of a 0.3...
Questions in other subjects:


Chemistry, 30.08.2019 20:00

History, 30.08.2019 20:00



Chemistry, 30.08.2019 20:00





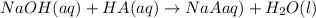
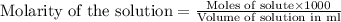 .....(1)
.....(1)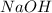 solution = 0.35 M
solution = 0.35 M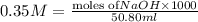
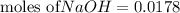
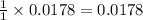 moles of HCl
moles of HCl