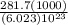Chemistry, 07.04.2020 22:28, mmassaro19
It takes 281.7 kJ of energy to remove 1 mole of electrons from the atoms on the surface of lithium metal. If lithium metal is irradiated with 261-nm light, what is the maximum kinetic energy the released electrons can have

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:50, KeiraDawn
An engineering team designs a new rocket that is faster and lighter than any other model being produced. however, the materials end up being so expensive that no company can afford to buy them. which step of the engineering process should have addressed this problem? a. know the background. b. evaluate the results. c. identify a need. d. do the work.
Answers: 2


Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2
Do you know the correct answer?
It takes 281.7 kJ of energy to remove 1 mole of electrons from the atoms on the surface of lithium m...
Questions in other subjects:


Chemistry, 07.07.2019 05:50


Biology, 07.07.2019 05:50

Social Studies, 07.07.2019 05:50

History, 07.07.2019 05:50

History, 07.07.2019 05:50

Biology, 07.07.2019 05:50

English, 07.07.2019 06:00

Social Studies, 07.07.2019 06:00


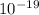 Joule
Joule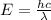 ------------ (1)
------------ (1)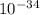 J s
J s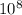
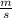
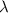 = 261 nm = 261 ×
= 261 nm = 261 × 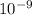 m
m