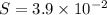Chemistry, 08.04.2020 00:09, chrismed2001
Lead(II) nitrate is added slowly to a solution that is 0.0800 M in Cl− ions. Calculate the concentration of Pb2+ ions (in mol / L) required to initiate the precipitation of PbCl2.(Ksp for PbCl2 is 2.40 ×10−4.)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, VictoriaRose520
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1


Chemistry, 22.06.2019 22:00, aliciaa101
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Do you know the correct answer?
Lead(II) nitrate is added slowly to a solution that is 0.0800 M in Cl− ions. Calculate the concentra...
Questions in other subjects:



Mathematics, 27.07.2019 14:20






Mathematics, 27.07.2019 14:20


![[Pb^{2+}]=3.9 \times 10^{-2}M](/tpl/images/0588/1239/e3715.png)
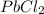 ⇄
⇄ 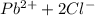
![Ip=[Pb^{2}][2Cl^-]^2=Ksp](/tpl/images/0588/1239/35efe.png)
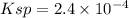
![[Pb^{2+}] = S](/tpl/images/0588/1239/e7380.png)
![[Cl^-]=2S](/tpl/images/0588/1239/41e82.png)
![Ksp=[Pb^{2+}]\times [Cl^-]^2](/tpl/images/0588/1239/02fd6.png)
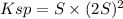
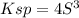
![S=\sqrt[3]{\frac{Ksp}{4} }](/tpl/images/0588/1239/e8aed.png)