

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 05:00, hjamya17
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2
Do you know the correct answer?
When the experiment was carried out, the temperature was 22 oC and atmospheric pressure was 1.007 at...
Questions in other subjects:



Mathematics, 13.03.2021 14:00


Mathematics, 13.03.2021 14:00

Mathematics, 13.03.2021 14:00

Mathematics, 13.03.2021 14:00

English, 13.03.2021 14:00

Mathematics, 13.03.2021 14:00


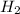 =4.16mol
=4.16mol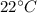 =273+22 k=295k
=273+22 k=295k



