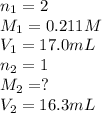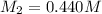Chemistry, 07.04.2020 21:20, jadbaubl1449
A titration reached the equivalence point when 17.0 mL of was added to of NaOH (aq) of unknown concentration. What is the concentration (M) of this unknown NaOH solution? H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + 2H2O(l)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, S4NCHEZ28
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2

Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 20:30, dinapaul424
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
Do you know the correct answer?
A titration reached the equivalence point when 17.0 mL of was added to of NaOH (aq) of unknown conce...
Questions in other subjects:


Chemistry, 10.03.2021 07:10


History, 10.03.2021 07:10

History, 10.03.2021 07:10




History, 10.03.2021 07:10

Mathematics, 10.03.2021 07:10


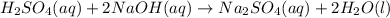
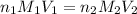
 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 
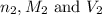 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.