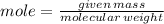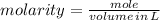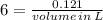To produce 40.0 g of silver chromate, you will need at least 23.4 g of potassium chromate in solution as a reactant. All you have on hand in the stock room is 5 L of a 6.00 M K2CrO4 solution. What volume of the solution is needed to give you the 23.4 g K2CrO4 needed for the reaction?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:50, kyleighmarie05
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2


Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
Do you know the correct answer?
To produce 40.0 g of silver chromate, you will need at least 23.4 g of potassium chromate in solutio...
Questions in other subjects:




History, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01



English, 16.10.2020 17:01


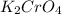 required=20.1 ml
required=20.1 ml