
Chemistry, 07.04.2020 21:50, hunteryolanda82
The Kb of weak base B is 1.0 x 10-6. A solution contains 0.75 M B and 0.25 M HB+Cl- (the salt of B with HCl). What is the pH of the solution after 0.05 mol NaOH is added to 1.0 L of the above solution?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:20, kkakk19
Calculate the enthalpy of the following reaction: 4 b (s) + 3 o2 (g) → 2 b2o3 (s) given the following pertinent information: (a) b2o3 (s) + 3 h2o (g) → 3 o2 (g) + b2h6 (g), δhoa = +2035 kj (b) 2 b (s) + 3 h2 (g) → b2h6 (g), δhob = +36 kj (c) h2 (g) + latex: \frac{1}{2} 1 2 o2 (g) → h2o (l), δhoc = −285 kj (d) h2o (l) → h2o (g), δhod = +44 kj
Answers: 3


Chemistry, 21.06.2019 22:00, toledanomariap43bxm
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 00:30, TMeansStupidity
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1
Do you know the correct answer?
The Kb of weak base B is 1.0 x 10-6. A solution contains 0.75 M B and 0.25 M HB+Cl- (the salt of B w...
Questions in other subjects:

Mathematics, 18.11.2020 03:20


English, 18.11.2020 03:20


English, 18.11.2020 03:20


Computers and Technology, 18.11.2020 03:20

History, 18.11.2020 03:20


Physics, 18.11.2020 03:20


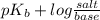
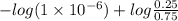
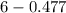
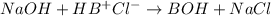
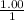 , [Salt] =
, [Salt] = 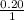
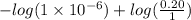 [/tex]
[/tex]



