
Which of the following statements is true? A. Lithium will react to give up an electron however that electron can only be used to make an anion of a metal B. Lithium is most easily oxidized of the metals listed on the activity series and therefore it will most easily give electrons to metal cations C. Lithium is least easily oxidized of the metals listed on the activity series and therefore it is least likely to give electrons to metal cations D. Lithium will only react with metal ions that require the addition of one electron because of the fact that lithium only gives up one electron

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, cordovamaria22
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2
Do you know the correct answer?
Which of the following statements is true? A. Lithium will react to give up an electron however that...
Questions in other subjects:

History, 18.03.2021 03:40

English, 18.03.2021 03:40

History, 18.03.2021 03:40


Mathematics, 18.03.2021 03:40






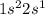 . It will attain stability by losing an electron.
. It will attain stability by losing an electron.



