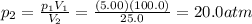Chemistry, 07.04.2020 20:36, sillyvanna
What is the final pressure when 100.0mL of a gas at 5.00 atm is compressed to 25.0mL at constant temperature?
(help! timed Test)
A.) 1.25 atm
B.) 500 atm
C.) 10.0 atm
D.) 20.0 atm

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, kev71
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1


Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2
Do you know the correct answer?
What is the final pressure when 100.0mL of a gas at 5.00 atm is compressed to 25.0mL at constant tem...
Questions in other subjects:

Mathematics, 24.02.2021 14:00


Mathematics, 24.02.2021 14:00




Mathematics, 24.02.2021 14:00




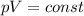
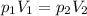
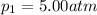 is the initial pressure of the gas
is the initial pressure of the gas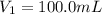 is the initial volume of the gas
is the initial volume of the gas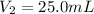 is the final volume of the gas
is the final volume of the gas