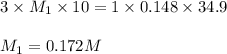Chemistry, 07.04.2020 19:27, KAITLYN007
A 25 ml volume of a sodium hydroxide solution requires 19.6 mL of a 0.189 M HCl acid for neutralization. A 10 mL volume of phosphoric acid solution requires 34.9 mL of the NaOH solution for complete neutralization. Calculate the concentration of the phosphoric acid solution.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, Lesquirrel
Acar tire has a pressure of 2.38 atm at 15.2 c. if the pressure inside reached 4.08 atm, the tire will explode. how hot would the tire have to get for this to happen? report the temperature in degrees celsius.
Answers: 2

Chemistry, 21.06.2019 22:00, pettygirl13
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 21.06.2019 23:00, orlando19882000
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 01:40, georgehall3027
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2
Do you know the correct answer?
A 25 ml volume of a sodium hydroxide solution requires 19.6 mL of a 0.189 M HCl acid for neutralizat...
Questions in other subjects:



Arts, 07.05.2021 01:10

Mathematics, 07.05.2021 01:10

Biology, 07.05.2021 01:10

Social Studies, 07.05.2021 01:10


Mathematics, 07.05.2021 01:10




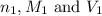 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 
 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.