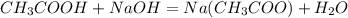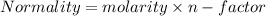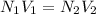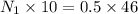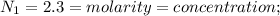Chemistry, 07.04.2020 19:22, genyjoannerubiera
In a laboratory experiment, a student titrates 10.0 mL of acetic acid, CH3COOH, solution with 0.5 M NaOH. The endpoint was reached when 46.00 mL of 0.50 M NaOH were delivery. What was the concentration of acetic acid in the solution

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, leilanimontes714
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
Do you know the correct answer?
In a laboratory experiment, a student titrates 10.0 mL of acetic acid, CH3COOH, solution with 0.5 M...
Questions in other subjects:

Mathematics, 23.04.2021 21:20



Mathematics, 23.04.2021 21:20