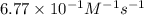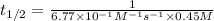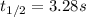Chemistry, 07.04.2020 18:45, ellycleland16
"The elementary reaction 2 NO2(g) → 2 NO(g) + O2(g) is second order in NO2 and the rate constant at 600 K is 6.77 × 10-1 M-1s-1. The reaction half-life at this temperature when [NO2]0 = 0.45 M is s."

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, kayleg907436
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1


Chemistry, 22.06.2019 19:00, Jasoncookies23
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
Do you know the correct answer?
"The elementary reaction 2 NO2(g) → 2 NO(g) + O2(g) is second order in NO2 and the rate constant at...
Questions in other subjects:


English, 10.06.2021 08:40


Mathematics, 10.06.2021 08:40



Mathematics, 10.06.2021 08:50



Mathematics, 10.06.2021 08:50


![t_{1/2}=\frac{1}{k\times [A_o]}](/tpl/images/0586/7504/a9a58.png)
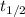 = half-life = ?
= half-life = ?![[A_o]](/tpl/images/0586/7504/dc622.png) = initial concentration = 0.45 M
= initial concentration = 0.45 M