

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 23:00, Mw3spartan17
What extra step distinguishes fermentation from glycolysis
Answers: 1


Chemistry, 23.06.2019 01:30, nikonee
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
Do you know the correct answer?
A sample of argon gas, Ar(g). is placed in a 3.80 L container at 320 K. The gas pressure is 0.496 at...
Questions in other subjects:


Mathematics, 15.08.2019 07:30

History, 15.08.2019 07:30


Mathematics, 15.08.2019 07:30


Mathematics, 15.08.2019 07:30



Mathematics, 15.08.2019 07:30


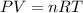 .......(1)
.......(1)




