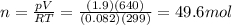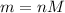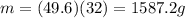Hi guys,
The questions says: "A single-patient hyperbaric chamber has a volume of 640 L. At a...

Chemistry, 07.04.2020 17:52, desiree3114
Hi guys,
The questions says: "A single-patient hyperbaric chamber has a volume of 640 L. At a temperature of 26 ∘C, how many grams of oxygen are needed to give a pressure of 1.9 atm?" It wants it up to 2 significant figures.
I did the problem a couple of times and it came up wrong. I'm not sure what I did wrong but any help would be appreciated.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, webbhlharryteach
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1


Chemistry, 22.06.2019 17:30, shookiegriffin
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1
Do you know the correct answer?
Questions in other subjects:



Social Studies, 05.05.2020 15:58

Social Studies, 05.05.2020 15:58



History, 05.05.2020 15:58



Mathematics, 05.05.2020 15:59


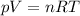
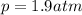 is the pressure of the gas
is the pressure of the gas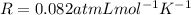 is the gas constant
is the gas constant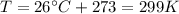 is the absolute temperature of the gas
is the absolute temperature of the gas