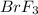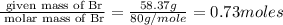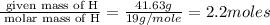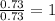Chemistry, 07.04.2020 17:21, kyrabrown33
A certain compound of bromine and fluorine is used to make UF6, which is an important chemical in the processing and reprocessing of nuclear fuel. The compound contains 58.37 mass percent bromine. What is its empirical formula

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 21:20, carlydays4403
The organs inside the body and how they function together
Answers: 3
Do you know the correct answer?
A certain compound of bromine and fluorine is used to make UF6, which is an important chemical in th...
Questions in other subjects:


Mathematics, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01

Chemistry, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01


Arts, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01

History, 20.09.2020 01:01