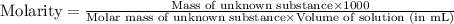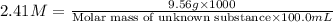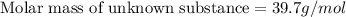A chemical test has determined the concentration of a solution of an unknown substance to be 2.41 M. a 100.0 mL volume of the solution is evaporated to dryness, leaving 9.56 g of crystals of the unknown solute. Calculate the molar mass of the unknown substance.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, mannster03
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2


Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
Do you know the correct answer?
A chemical test has determined the concentration of a solution of an unknown substance to be 2.41 M....
Questions in other subjects:


Mathematics, 31.03.2020 07:04

Chemistry, 31.03.2020 07:04

Biology, 31.03.2020 07:04


Mathematics, 31.03.2020 07:05

Computers and Technology, 31.03.2020 07:05

Mathematics, 31.03.2020 07:05


Mathematics, 31.03.2020 07:05