HX(aq) + H2O(l) → H3O+(aq) + X−(aq)
Based on the equation, HX would be classified as A) an aci...

Chemistry, 07.04.2020 06:38, isabelvaldez123
HX(aq) + H2O(l) → H3O+(aq) + X−(aq)
Based on the equation, HX would be classified as A) an acid, because it accepts a proton
B) a base, because it accepts a proton. C) a base, because it donates a proton D) an acid, because it donates a proton

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, palcochran1313
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1


Do you know the correct answer?
Questions in other subjects:








History, 02.08.2019 03:00

Physics, 02.08.2019 03:00

History, 02.08.2019 03:00


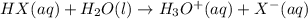
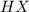 is loosing a proton, thus it is considered as an acid and after losing a proton, it forms
is loosing a proton, thus it is considered as an acid and after losing a proton, it forms 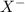 which is a conjugate base.
which is a conjugate base.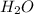 is gaining a proton, thus it is considered as a base and after gaining a proton, it forms
is gaining a proton, thus it is considered as a base and after gaining a proton, it forms 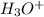 which is a conjugate acid.
which is a conjugate acid.



