
The breathalyzer test utilizes the reaction between the dichromate ion and ethanol to produce acetic acid. Balanced equation for this process is given below. How much acetic acid can be produced from a mixture containing an excess of dichromate ion and 1.37 × 10−1 g of ethanol?
16H+ + 2Cr2O72− + 3CH3CH2OH → 3CH3COOH + 4Cr3+ + 11H2O

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, mamabates181981
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 12:40, valenzueladomipay09u
How does concentration affect reaction rate
Answers: 2

Chemistry, 22.06.2019 13:30, ayoismeisalex
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1
Do you know the correct answer?
The breathalyzer test utilizes the reaction between the dichromate ion and ethanol to produce acetic...
Questions in other subjects:


Mathematics, 24.11.2019 08:31


English, 24.11.2019 08:31

Biology, 24.11.2019 08:31

History, 24.11.2019 08:31



Health, 24.11.2019 08:31


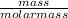
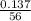 = 0.0025moles
= 0.0025moles 



