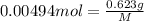A 0.623 g sample of a monoprotic acid is dissolved in water and titrated with 0.260 M KOH.
Wh...

Chemistry, 07.04.2020 04:53, granthazenp5e9mj
A 0.623 g sample of a monoprotic acid is dissolved in water and titrated with 0.260 M KOH.
What is the molar mass of the acid if 19.0 mL of the KOH solution is required to neutralize the sample?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, awdadaddda
How air particles exert a pressure on the inside of the balloon
Answers: 1


Do you know the correct answer?
Questions in other subjects:


Mathematics, 25.06.2020 09:01

Mathematics, 25.06.2020 09:01

Health, 25.06.2020 09:01


History, 25.06.2020 09:01

Mathematics, 25.06.2020 09:01


English, 25.06.2020 09:01


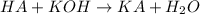
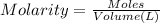
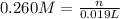
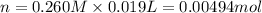
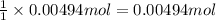 of HA
of HA