
Chemistry, 07.04.2020 04:59, tnbankspines
Using your value of Ksp, and starting with an equilibrium system consisting of a saturated solution of calcium hydroxide, predict whether the solubility will increase or decrease it you add a. A. 5.00 mL of 0.100 M HNO3 Ksp = 2.966610-5 b. 5.00 mL of 0.100 M NaOH C. 5.00 mL of 0.100 M Ca(NO3)2

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, milesjreece3939
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 04:30, akeemedwards12
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
Do you know the correct answer?
Using your value of Ksp, and starting with an equilibrium system consisting of a saturated solution...
Questions in other subjects:

Computers and Technology, 30.07.2019 13:00





Mathematics, 30.07.2019 13:00


Mathematics, 30.07.2019 13:00



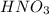 Solubility will increase.
Solubility will increase.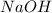 Solubility will decrease.
Solubility will decrease.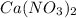 Solubility will decrease.
Solubility will decrease.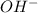 are consumed due to it and the system would respond by reaction shifting towards creation of more
are consumed due to it and the system would respond by reaction shifting towards creation of more 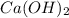 precipitating back. Hence solubility decreases.
precipitating back. Hence solubility decreases.
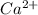 which will be countered by reaction shifting to side that decreases increased
which will be countered by reaction shifting to side that decreases increased 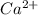 , which happens by some
, which happens by some 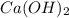 precipitating back. Hence solubility decreases.
precipitating back. Hence solubility decreases.



