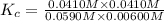Chemistry, 07.04.2020 03:15, sethlynn2003
Calculate the equilibrium constant for the reaction using the balanced chemical equation and the concentrations of the substances at equilibrium.
Use the
appropriate significant figures in reporting the answers.
CO(g) + H2O(g) ⇌ CO2(g) + H2(g) [CO] = 0.0590 M; [H2O] = 0.00600 M;
[CO2] = 0.0410 M; [H2] = 0.0410 M
K =-

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, aidengalvin20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 04:30, logan12345677885675
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 09:00, hellodarkness14
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3
Do you know the correct answer?
Calculate the equilibrium constant for the reaction using the balanced chemical equation and the con...
Questions in other subjects:



History, 05.11.2020 19:10




Mathematics, 05.11.2020 19:10

Biology, 05.11.2020 19:10



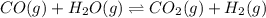
![[CO]=0.0590 M,[H_2O]=0.00600 M](/tpl/images/0585/8718/63397.png)
![[CO_2]=0.0410 M,[H_2]=0.0410 M](/tpl/images/0585/8718/efcda.png)
![K_c=\frac{[CO_2][H_2]}{[CO][H_2O]}](/tpl/images/0585/8718/fbbde.png)