
Chemistry, 07.04.2020 02:35, uglyturtle15
Consider the reaction of magnesium metal with hydrochloric acid to produce magnesium chloride and hydrogen gas. If 3.29 mol of magnesium and 3.29 mol of hydrochloric acid are reacted, how many moles of excess reactant are left over?
a. 6.58 mol
b. 3.29 mol
c. 2.47 mol
d. 40.0 mol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, WhiteWinterRose
What is the chemical formula of the following compound
Answers: 3


Chemistry, 22.06.2019 09:00, stelllllllllllllllla
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
Do you know the correct answer?
Consider the reaction of magnesium metal with hydrochloric acid to produce magnesium chloride and hy...
Questions in other subjects:

Social Studies, 16.01.2021 16:30

Mathematics, 16.01.2021 16:30









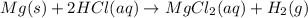
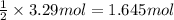 of magnesium metal
of magnesium metal



