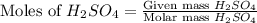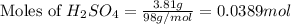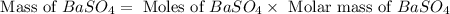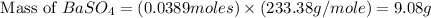Chemistry, 07.04.2020 01:26, eaalvarezelisa01
For the following reaction, 3.81 grams of sulfuric acid are mixed with excess barium hydroxide. The reaction yields 8.45 grams of barium sulfate.
barium hydroxide (aq) + sulfuric acid (aq) ---> barium sulfate (s) + water (l)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, tae8002001
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2


Chemistry, 23.06.2019 14:30, jenorajordan5387
An atom of element x has one more shell of electrons than an atom of beryllium, but it has one less valance electron than beryllium. which element is x
Answers: 1
Do you know the correct answer?
For the following reaction, 3.81 grams of sulfuric acid are mixed with excess barium hydroxide. The...
Questions in other subjects:







Physics, 14.08.2020 15:01



Computers and Technology, 14.08.2020 15:01


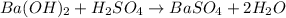
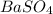 is, 9.08 grams.
is, 9.08 grams.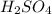 = 3.81 g
= 3.81 g