
Wine goes bad soon after opening because the ethanol dissolved in it reacts with oxygen gas to form water and aqueous acetic acid , the main ingredient in vinegar. Calculate the moles of "oxygen" needed to produce "0.085" mol of "water". Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 23:00, brianfranklin17
What is the correct lewis dot structure for arsenic?
Answers: 2

Chemistry, 23.06.2019 08:00, kathrynpuppies201716
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0.00°c and a volume of 0.950 l? which equation should you use? p2= p1v1t2/t1v2what is the pressure of the gas? 3.92 atm these are the answers
Answers: 1
Do you know the correct answer?
Wine goes bad soon after opening because the ethanol dissolved in it reacts with oxygen gas to form...
Questions in other subjects:

History, 20.05.2021 01:30


English, 20.05.2021 01:30





Mathematics, 20.05.2021 01:30


Mathematics, 20.05.2021 01:30


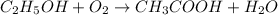
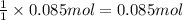 of oxygen gas
of oxygen gas 



