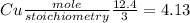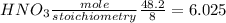Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, kellypechacekoyc1b3
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 23.06.2019 10:10, Kennethabrown09
In a covalent bond, two atoms are held together by the attraction between . the number of covalent bonds that an atom can form depends on the number of in the atom.
Answers: 2
Do you know the correct answer?
If 12.4 moles of Cu and 48.2 moles of HNO3 are allowed to react, how many moles of excess reactant w...
Questions in other subjects:

Biology, 13.12.2021 03:50

Mathematics, 13.12.2021 03:50


Mathematics, 13.12.2021 03:50

History, 13.12.2021 03:50

Mathematics, 13.12.2021 03:50


Mathematics, 13.12.2021 03:50