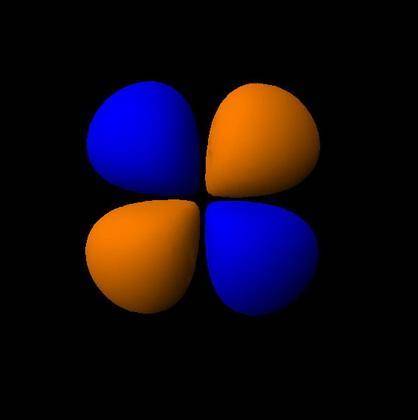Chemistry, 07.04.2020 00:47, glowbaby123
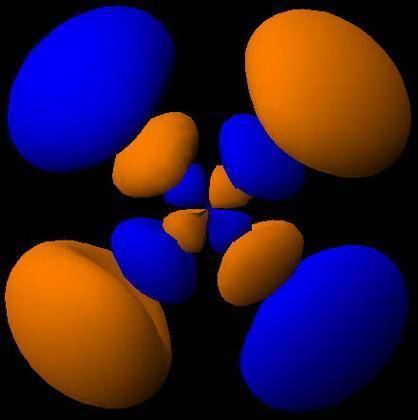
How would the dx2−y2 orbital in the n=5 shell compare to the dx2−y2 orbital in the n=3 shell?
Determine which statement is true or false.
The orbital in the n = 5 shell is bigger than the orbital in the n = 3 shell.
The value of l would increase by 2 for the n = 5 shell.
The value of l for both orbitals would be the same.
The orientation of the n = 5 orbital would be rotated 45∘ along the xy plane.
The mâ„“ value for both orbitals would be the same.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, pinkycupcakes3oxbqhx
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2


Chemistry, 22.06.2019 21:20, jordan2875
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 23.06.2019 05:40, MyChannelBruh6896
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
Do you know the correct answer?
How would the dx2−y2 orbital in the n=5 shell compare to the dx2−y2 orbital in the n=3 shell?
Questions in other subjects:

Spanish, 27.05.2021 22:20


English, 27.05.2021 22:20






Mathematics, 27.05.2021 22:20