
Chemistry, 06.04.2020 22:19, birdwithpurpleboots
Consider a mixture of air and gasoline vapor in a cylinder with a piston. The original volume is 0.09 L. If the combustion of this mixture releases 900. Jof energy, to what volume will the gases expand against a constant pressure of 670. torr if all the energy of combustion is converted into work to push back the piston? (1 atm = 760 torr, 1 L atm = 101.325 J) a. 10.00 L b. 10.17 L c. 1.47 L d. 1.34L

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, rscott2649
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
Do you know the correct answer?
Consider a mixture of air and gasoline vapor in a cylinder with a piston. The original volume is 0.0...
Questions in other subjects:




English, 02.03.2021 18:50

Arts, 02.03.2021 18:50

Mathematics, 02.03.2021 18:50

English, 02.03.2021 18:50

Mathematics, 02.03.2021 18:50



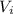 = 0.09 L, P = 670 torr
= 0.09 L, P = 670 torr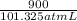
 atm
atm
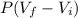
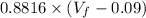
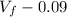 = 10.08
= 10.08
 = 10.17 L
= 10.17 L




