
Answer the following questions about the solubility of AgCl(s). The value of Ksp for AgCl(s) is 1.8 × 10−10.
Calculate the value of [Ag+] in a saturated solution of AgCl in distilled water.
The concentration of Cl−(aq) in seawater is 0.54 M.
Calculate the molar solubility of AgCl(s) in seawater.
Explain why AgCl(s) is less soluble in seawater than in distilled water.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, ayoismeisalex
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1



Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1
Do you know the correct answer?
Answer the following questions about the solubility of AgCl(s). The value of Ksp for AgCl(s) is 1.8...
Questions in other subjects:



Business, 09.10.2019 15:10

English, 09.10.2019 15:10



History, 09.10.2019 15:20




 M.
M.
 = x
= x  x
x

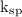 = b
= b 





