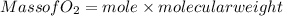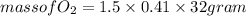Chemistry, 06.04.2020 18:21, roseemariehunter12
Potassium superoxide, KO2, is often used in oxygen masks (such as those used by firefighters) because KO2 reacts with CO2 to release molecular oxygen. Experiments indicate that 2 mol of KO2(s) react with each mole of CO2(g).
(a) The products of the reaction are K2CO3(s) and O2(g). Write a balanced equation for the reaction between KO2(s) and CO2(g).
(b) Indicate the oxidation number for each atom involved in the reaction in part (a). What elements are being oxidized and reduced?
(c) What mass of KO2(s) is needed to consume 18.0 g CO2(g)? What mass of O2(g) is produced during this reaction?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Do you know the correct answer?
Potassium superoxide, KO2, is often used in oxygen masks (such as those used by firefighters) becaus...
Questions in other subjects:




Mathematics, 18.05.2021 06:00


Mathematics, 18.05.2021 06:00


Mathematics, 18.05.2021 06:00


Mathematics, 18.05.2021 06:00


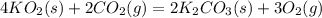
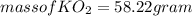 and
and 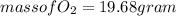
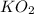 need 2 moles of
need 2 moles of  for complete reaction i.e. mole
for complete reaction i.e. mole 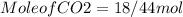
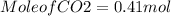
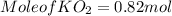
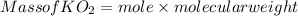
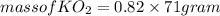
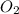 is produced
is produced 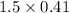 mole of
mole of