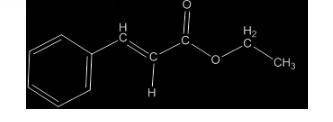
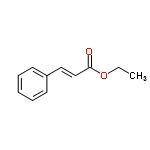
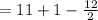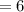A compound, C11H12O2, has an IR spectrum showing a peak at 1710 cm-1. Its 1H NMR spectrum has peaks at delta 1.3 (3 H, triplet), 4.3 (2 H, quartet), 6.5 (1 H, doublet), 7.4-7.6 (5 H, multiplet), and 7.7 (1 H, doublet). Draw its structure in the window below. You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. Do not include lone pairs in your answer. They will not be considered in the grading.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, YoVeoAnime
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1

Chemistry, 22.06.2019 23:00, lulprettyb
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 23.06.2019 00:00, PineappleDevil889
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
Do you know the correct answer?
A compound, C11H12O2, has an IR spectrum showing a peak at 1710 cm-1. Its 1H NMR spectrum has peaks...
Questions in other subjects:

Spanish, 18.12.2020 04:10




Spanish, 18.12.2020 04:10

Mathematics, 18.12.2020 04:10



English, 18.12.2020 04:10

Arts, 18.12.2020 04:10