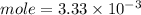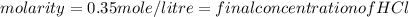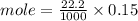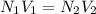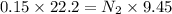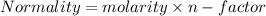Chemistry, 06.04.2020 18:02, hntnhtthnyt
Two burettes are set up. The first contains 0.15M NaOH and the second contains an HCl solution of unknown concentration. HCl is dispensed into the reaction flask and Phenolphthalein is added as an indicator. At the end of the titration, 22.2ml of NaOH and 9.45ml HCl were used.
Required:
A. Write a balanced equation for the Acid-Base reaction.
B. What is the total volume of NaOH used in the titration?
C. How many moles of NaOH was used?
D. What is the total volume of HCl used in the titration?
E. What is the final concentration of the HCl solution?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 21:30, rileydavidharless
Which substance can be broken down by chemical means
Answers: 1

Chemistry, 23.06.2019 00:30, ejuarez2020
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

Chemistry, 23.06.2019 04:00, ayoismeisjjjjuan
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
Do you know the correct answer?
Two burettes are set up. The first contains 0.15M NaOH and the second contains an HCl solution of un...
Questions in other subjects:



English, 18.03.2021 19:00


Mathematics, 18.03.2021 19:00



Mathematics, 18.03.2021 19:00

English, 18.03.2021 19:00