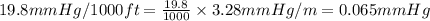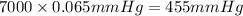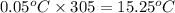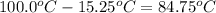The vapor pressure of a substance describes how readily molecules at the surface of the substance enter the gaseous phase. At the boiling point of a liquid, the liquid's vapor pressure is equal to or greater than the atmospheric pressure exerted on the surface of the liquid. Since the atmospheric pressure at higher elevations is lower than at sea level, the boiling point of water decreases as the elevation increases. The atmospheric pressure at sea level is 760 mmHg. This pressure decreases by 19.8 mmHg for every 1000-ft increase in elevation. Elevation Pressure0 m 760 mmHg1000 m 695 mmHg2000 m 630 mmHgThe boiling point of water decreases 0.05 ?C for every 1 mmHg drop in atmospheric pressure. A) What is the boiling point of water at an elevation of 7000m ?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, anamaliiow
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 04:30, jocelynmarquillo1
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Do you know the correct answer?
The vapor pressure of a substance describes how readily molecules at the surface of the substance en...
Questions in other subjects:

Computers and Technology, 02.12.2021 01:30


Mathematics, 02.12.2021 01:30

Spanish, 02.12.2021 01:30



Mathematics, 02.12.2021 01:30



Chemistry, 02.12.2021 01:30


 meter
meter