
Chemistry, 06.04.2020 11:30, justice808
4. Calculate the number of liters of oxygen gas needed to produce 15.0 liters of dinitrogen trioxide. Assume all gases are at the same conditions of temperature and pressure.
2N2(g) + 3O2(g) 2N2O3(g)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, dustinquiz255
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3


Chemistry, 22.06.2019 15:20, Tringirl233
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
Do you know the correct answer?
4. Calculate the number of liters of oxygen gas needed to produce 15.0 liters of dinitrogen trioxide...
Questions in other subjects:

Mathematics, 24.04.2020 22:44

Geography, 24.04.2020 22:44

Mathematics, 24.04.2020 22:44


Mathematics, 24.04.2020 22:44

Mathematics, 24.04.2020 22:44


Mathematics, 24.04.2020 22:44



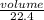
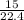 = 0.67moles
= 0.67moles 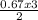 = 1.01 moles of O₂
= 1.01 moles of O₂



