

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 11:30, melissalopez12
Place the following substances in order of ph from lowest ph to highest. a. neutral compounds, bases, acids b. acids, neutral compounds, bases c. bases, acids, neutral compounds d. bases, neutral compounds, acids
Answers: 1


Do you know the correct answer?
The enthalpy change for converting 10.0 g of ice at -25.0 °c to water at 90.0 °c is kj. the specifi...
Questions in other subjects:

Computers and Technology, 08.12.2020 23:50


Social Studies, 08.12.2020 23:50

French, 08.12.2020 23:50

Mathematics, 08.12.2020 23:50

Mathematics, 08.12.2020 23:50



Mathematics, 08.12.2020 23:50

Mathematics, 08.12.2020 23:50


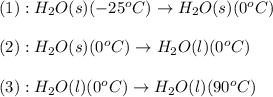
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]](/tpl/images/0474/9125/5cd06.png)
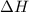 = enthalpy change = ?
= enthalpy change = ?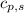 = specific heat of solid water =
= specific heat of solid water = 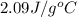
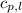 = specific heat of liquid water =
= specific heat of liquid water = 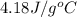
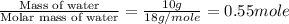
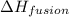 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole![\Delta H=[10g\times 4.18J/gK\times (0-(-25))^oC]+0.55mole\times 6010J/mole+[10g\times 2.09J/gK\times (90-0)^oC]](/tpl/images/0474/9125/4de79.png)
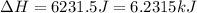 (1 KJ = 1000 J)
(1 KJ = 1000 J)



