

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:30, falishaduncanovmtz2
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 14:30, joejoefofana
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀ pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4. 0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 17:20, alexis3060
How do you know when a chemical reaction has occurred
Answers: 1
Do you know the correct answer?
Exactly 50.0mL of HOCl solution of unknown concentration was titrated with 0.100 mol NaOH. An end po...
Questions in other subjects:




Physics, 23.07.2019 20:30

Biology, 23.07.2019 20:30


Mathematics, 23.07.2019 20:30



World Languages, 23.07.2019 20:30


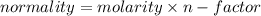
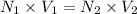
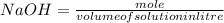
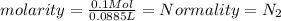
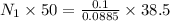
 ;
;



