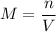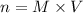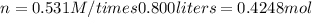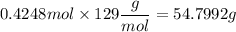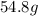Chemistry, 05.04.2020 17:39, laceysmith2i023
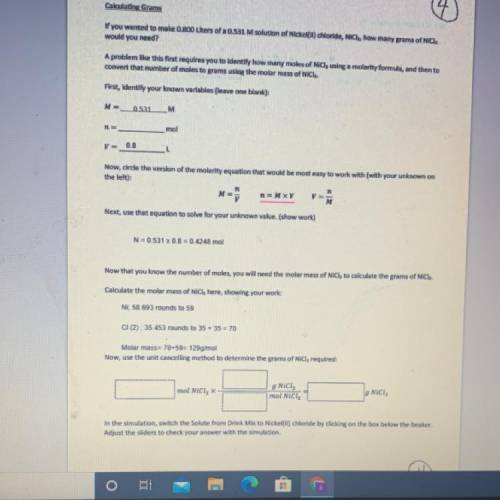
If you wanted to make 0.800 Liters of a 0.531 M solution of Nickel(II) chloride, NiCl2, how many grams of NiCIZ
would you need? Use the unit canceling method to determine the grams of NiCl2
Molar mass= 129g/mol. Moles = 0.4248
.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:20, johnkings140
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1

Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 22.06.2019 22:30, brianna5626
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
Do you know the correct answer?
If you wanted to make 0.800 Liters of a 0.531 M solution of Nickel(II) chloride, NiCl2, how many gra...
Questions in other subjects:

Biology, 29.09.2019 12:00

Spanish, 29.09.2019 12:00




History, 29.09.2019 12:00

Social Studies, 29.09.2019 12:00