
Chemistry, 04.04.2020 14:28, shaydog6353
Consider the reaction between 15.0 mL of a 1.00 M aqueous solution of AgNO3 and 10.0 mL of a 1.00 M aqueous solution of K2CrO4. When these react, a precipitate is observed. What is present in solution after the reaction is complete

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, codeyhatch142
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Do you know the correct answer?
Consider the reaction between 15.0 mL of a 1.00 M aqueous solution of AgNO3 and 10.0 mL of a 1.00 M...
Questions in other subjects:


Computers and Technology, 06.12.2021 19:30






Mathematics, 06.12.2021 19:30


Mathematics, 06.12.2021 19:30


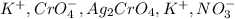
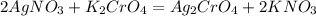
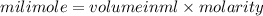
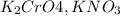 are soluble in water so it will dissociate in the solution
are soluble in water so it will dissociate in the solution



