
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming of natural gas, in a two-step process. In the first step, nitrogen and hydrogen react to form ammonia: (g)(g)(g) In the second step, ammonia and oxygen react to form nitric acid and water: (g)(g)(g)(g) Write the net chemical equation for the production of nitric acid from nitrogen, hydrogen and oxygen. Be sure your equation is balanced.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Do you know the correct answer?
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prep...
Questions in other subjects:

Mathematics, 08.12.2020 01:00


Mathematics, 08.12.2020 01:00




World Languages, 08.12.2020 01:00




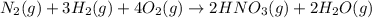
 (1)
(1)

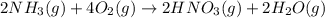 (2)
(2)



