Chemistry, 04.04.2020 11:34, akatsionis25
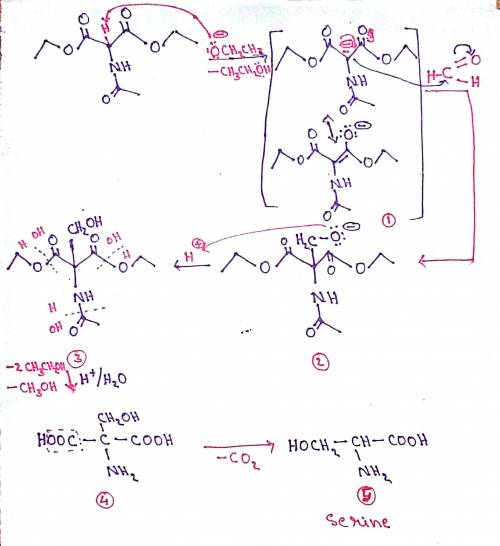
A variation of the acetamidomalonate synthesis can be used to synthesize serine. The process involves the following steps: Ethoxide ion deprotonates diethyl acetamidomalonate, forming enolate anion 1; Enolate anion 1 makes a nucleophilic attack on formaldehyde, forming tetrahedral intermediate 2; Protonation of the oxyanion forms alcohol 3; Acid hydrolysis yields dicarboxyamino alcohol 4; Decarboxylation leads to the final amino acid. Write out the mechanism on a separate sheet of paper, and then draw the structure of dicarboxyamino alcohol 4.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, dontcareanyonemo
Select the correct answer. what is the nature of the se-cl bond in a molecule of selenium chloride (secl2) if the electronegativity value of selenium is 2.55 and that of chlorine is 3.16?
Answers: 3

Chemistry, 21.06.2019 19:00, anonymous176
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 22.06.2019 22:30, medinajocelyn45
Which compound most likely has the greatest bond energy?
Answers: 2
Do you know the correct answer?
A variation of the acetamidomalonate synthesis can be used to synthesize serine. The process involve...
Questions in other subjects:




English, 12.03.2021 14:00

Mathematics, 12.03.2021 14:00

Biology, 12.03.2021 14:00

Social Studies, 12.03.2021 14:00


Mathematics, 12.03.2021 14:00