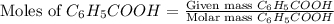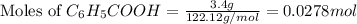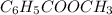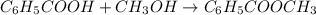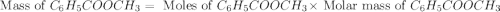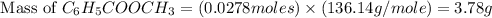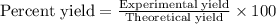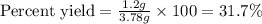Chemistry, 04.04.2020 11:01, meganwintergirl
A reaction was performed in which 3.4 g of benzoic acid was reacted with excess methanol to make 1.2 g of methyl benzoate. Calculate the theoretical yield and percent yield for this reaction.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, mimibear2932
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 23.06.2019 01:30, ayoismeisalex
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1

Chemistry, 23.06.2019 03:00, BeeShyanne
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
Do you know the correct answer?
A reaction was performed in which 3.4 g of benzoic acid was reacted with excess methanol to make 1.2...
Questions in other subjects:

Spanish, 20.10.2019 08:20







SAT, 20.10.2019 08:20



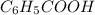 = 3.4 g
= 3.4 g