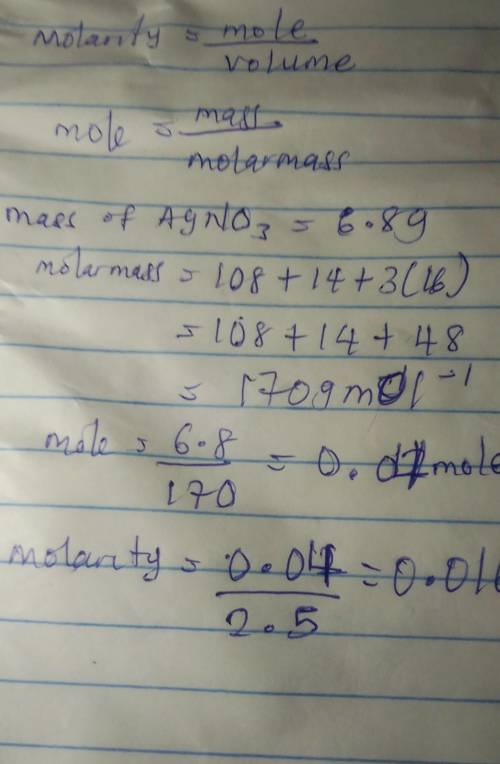Chemistry, 04.04.2020 11:03, capricorn0115
Calculate the molarity of a solution prepared by dissolving 6.80 grams of AgNO3 in 2.50 liters of solution.
SHOW WORK:)

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:10, hannah2757
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
Do you know the correct answer?
Calculate the molarity of a solution prepared by dissolving 6.80 grams of AgNO3 in 2.50 liters of so...
Questions in other subjects:

English, 29.10.2019 22:31



Mathematics, 29.10.2019 22:31

History, 29.10.2019 22:31

Mathematics, 29.10.2019 22:31

English, 29.10.2019 22:31