Chemistry, 04.04.2020 10:53, Cooldude3966
A simple equation relates the standard free‑energy change, ΔG∘′, to the change in reduction potential. ΔE0′. ΔG∘′ = −nFΔE0′ The n represents the number of transferred electrons, and F is the Faraday constant with a value of 96.48 kJ⋅mol^(−1)⋅V^(−1). Use the standard reduction potentials provided to determine the standard free energy released by reducing O2 with FADH2. FADH2 + 1/2O2 → FAD + H2O
given that the standard reduction potential for the reduction of oxygen to water is +0.82 V and for the reduction of FAD to FADH2 is +0.03 V.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, valdezlizbeth6652
Why is carbon ideal for making different compounds?
Answers: 2

Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 17:40, aaliyahthomas37
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
Do you know the correct answer?
A simple equation relates the standard free‑energy change, ΔG∘′, to the change in reduction potentia...
Questions in other subjects:

English, 20.09.2020 04:01


Biology, 20.09.2020 04:01


Engineering, 20.09.2020 04:01



Arts, 20.09.2020 04:01


History, 20.09.2020 04:01


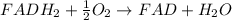
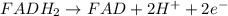
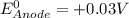
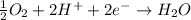
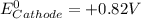
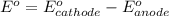
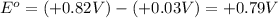
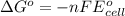
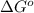 = standard free energy = ?
= standard free energy = ?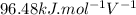
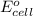 = standard electrode potential of the cell = 0.79 V
= standard electrode potential of the cell = 0.79 V