
Chemistry, 04.04.2020 11:09, quickestlearner5694
As an approximation, we can assume that proteins exist either in the native (or physiologically functioning) state or the denatured state. The standard molar enthalpy and entropy of the denaturation of a certain protein are 545 kJ·mol−1 and 1.55 kJ·K−1·mol−1, respectively. Comment on the signs and magnitudes of these quantities.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, joelpimentel
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 10:00, valdezlizbeth6652
Why is carbon ideal for making different compounds?
Answers: 2

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Do you know the correct answer?
As an approximation, we can assume that proteins exist either in the native (or physiologically func...
Questions in other subjects:


Geography, 01.04.2021 22:10




Mathematics, 01.04.2021 22:10

Mathematics, 01.04.2021 22:10

English, 01.04.2021 22:10

Mathematics, 01.04.2021 22:10


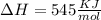
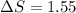
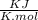
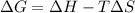
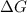 is negative
is negative 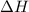 and
and 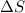 are positive, so reaction is spontaneous at
are positive, so reaction is spontaneous at 



