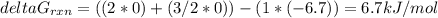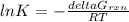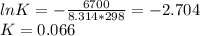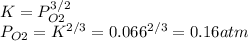Chemistry, 04.04.2020 09:43, emmarieasimon
Consider the decomposition of a metal oxide to its elements, where M represents a generic metal. M 3 O 4 ( s ) − ⇀ ↽ − 3 M ( s ) + 2 O 2 ( g ) What is the standard change in Gibbs energy for the reaction, as written, in the forward direction? Δ G ∘ rxn = kJ/mol What is the equilibrium constant of this reaction, as written, in the forward direction at 298 K? K = What is the equilibrium pressure of O2(g) over M(s) at 298 K? P O 2 = atm

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
Do you know the correct answer?
Consider the decomposition of a metal oxide to its elements, where M represents a generic metal. M 3...
Questions in other subjects:

Mathematics, 19.10.2019 23:30


Mathematics, 19.10.2019 23:30



English, 19.10.2019 23:30



Arts, 19.10.2019 23:30

Social Studies, 19.10.2019 23:30