
Chemistry, 04.04.2020 10:06, bluebunny1231999
Calculate the pH for the following weak acid. A solution of HCOOH has 0.12M HCOOH at equilibrium. The Ka for HCOOH is 1.8×10−4. What is the pH of this solution at equilibrium?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, happy121906
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 07:30, SchoolFirst9811
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н, о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 17:00, BREBRE8932
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
Do you know the correct answer?
Calculate the pH for the following weak acid. A solution of HCOOH has 0.12M HCOOH at equilibrium. Th...
Questions in other subjects:




Mathematics, 28.10.2020 16:30






Mathematics, 28.10.2020 16:30


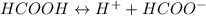
![\Ka= \frac{[H^+][HCOO^-]}{[HCOOH]}](/tpl/images/0582/0861/d7b9e.png)
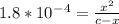
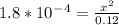
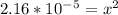
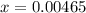
![[H^+] .](/tpl/images/0582/0861/74f2d.png)
![[H^+] = 0.00465 M](/tpl/images/0582/0861/817d9.png)
![pH=-log[H^+]](/tpl/images/0582/0861/15713.png)
![K_{a} = \frac{[H^{+} ][HCOO^{-} ]}{[HCOOH]}](/tpl/images/0582/0861/8c4ca.png)
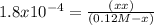
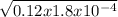 = 4.65 x 10⁻³
= 4.65 x 10⁻³



