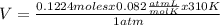Chemistry, 04.04.2020 01:45, michaelgold1
The equation for the metabolic breakdown of glucose (C6H12O6) is the same as the equation for the combustion of glucose in air: C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l) Calculate the volume of CO2 produced at 37°C and 1.00 atm when 3.68 g of glucose is used up in the reaction.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:40, larkinc2946
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 01:00, williedenmark42
What is the most common form of matter in the universe
Answers: 2

Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
Do you know the correct answer?
The equation for the metabolic breakdown of glucose (C6H12O6) is the same as the equation for the co...
Questions in other subjects:

English, 11.03.2020 09:10

English, 11.03.2020 09:10




Mathematics, 11.03.2020 09:11


Mathematics, 11.03.2020 09:12



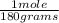 = 0.0204 moles
= 0.0204 moles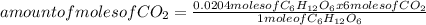
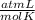 T= 37 C= 310 K (being 0 C= 273 K)
T= 37 C= 310 K (being 0 C= 273 K)