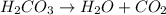Chemistry, 30.10.2019 08:31, jngonzo1226
When magnesium carbonate, mgco2, reacts with nitric acid, hno3, it forms magnesium nitrate, mg(no3)2, and carbonic acid, h2co3. carbonic acid then breaks down into water and carbon dioxide. which two types of reactions take place in this process?
a.) synthesis and decomposition
b.) single-replacement and combustion
c.) double-replacement and decomposition
d.) double-replacement and combustion

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Do you know the correct answer?
When magnesium carbonate, mgco2, reacts with nitric acid, hno3, it forms magnesium nitrate, mg(no3)2...
Questions in other subjects:


Mathematics, 27.05.2020 23:02


History, 27.05.2020 23:02




Mathematics, 27.05.2020 23:02


Chemistry, 27.05.2020 23:02


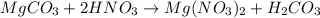 : is a double displacement reaction in which ion exchange takes place.
: is a double displacement reaction in which ion exchange takes place.