N
evious
Next →
Post Test: Chemical Reactions
Submit Test
Reader Tools...

Chemistry, 03.04.2020 02:03, hjukhvfd5723
N
evious
Next →
Post Test: Chemical Reactions
Submit Test
Reader Tools
Info
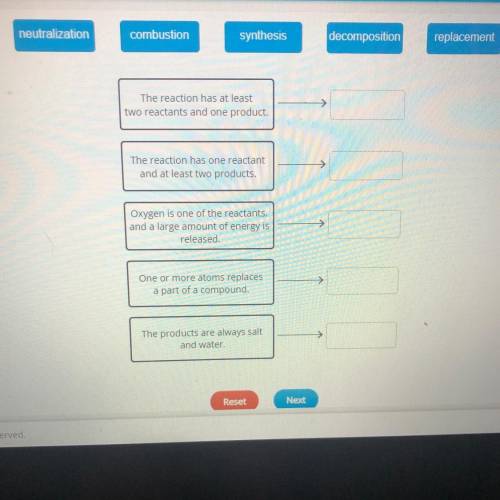
neutralization
combustion
synthesis
decomposition
replacement
The reaction has at least
two reactants and one product.
-
The reaction has one reactant
and at least two products.
Oxygen is one of the reactants.
and a large amount of energy is
released.
One or more atoms replaces
a part of a compound.
The products are always salt
and water

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, eamccoy1
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 07:20, JKINGblackstar3502
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 12:00, BakerElsie02
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 20:30, huangjianhe135
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
Do you know the correct answer?
Questions in other subjects:




History, 09.11.2020 01:00

Mathematics, 09.11.2020 01:00




Mathematics, 09.11.2020 01:00







