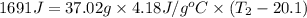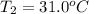Chemistry, 02.04.2020 01:26, kevinkingpin
What is the final temperature of the solution formed when 1.52 g of NaOH is added to 35.5 g of water at 20.1 °C in a calorimeter? NaOH (s) → Na+ (aq) + OH– (aq) ∆H = -44.5 kJ/mol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, tgraveslaylay2743
Bose-einstein condensation occurs at what temperature?
Answers: 2

Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2


Chemistry, 22.06.2019 17:00, brownvester44
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
Do you know the correct answer?
What is the final temperature of the solution formed when 1.52 g of NaOH is added to 35.5 g of water...
Questions in other subjects:

Mathematics, 20.01.2021 17:40


Mathematics, 20.01.2021 17:40





History, 20.01.2021 17:40

Mathematics, 20.01.2021 17:40


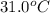
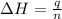
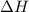 = enthalpy change = -44.5 kJ/mol
= enthalpy change = -44.5 kJ/mol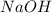 = 1.52 g
= 1.52 g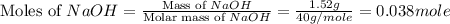
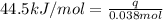
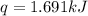
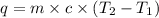
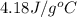
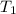 = initial temperature =
= initial temperature = 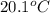
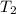 = final temperature = ?
= final temperature = ?