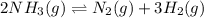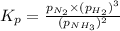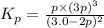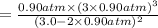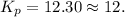Chemistry, 02.04.2020 01:21, leannaadrian
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a 500. ML flask with 3.0 atm of ammonia gas, and when the mixture has come to equilibrium measures the amount of nitrogen gas to be 0.90 atm . Calculate the pressure equilibrium constant for the decomposition of ammonia at the final temperature of the mixture. Round your answer to 2 significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, jusicca1109
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 23.06.2019 07:00, bree6754
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3


Chemistry, 23.06.2019 16:50, alosnomolina1122
Consider the balanced equation below. pcl3+cl2-> pcl5 what is the mole ratio of pcl3 to pcl5
Answers: 1
Do you know the correct answer?
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studyin...
Questions in other subjects:

History, 09.09.2019 12:10

History, 09.09.2019 12:10

Mathematics, 09.09.2019 13:10


English, 09.09.2019 13:10


Social Studies, 09.09.2019 13:10

Mathematics, 09.09.2019 13:10

English, 09.09.2019 13:10